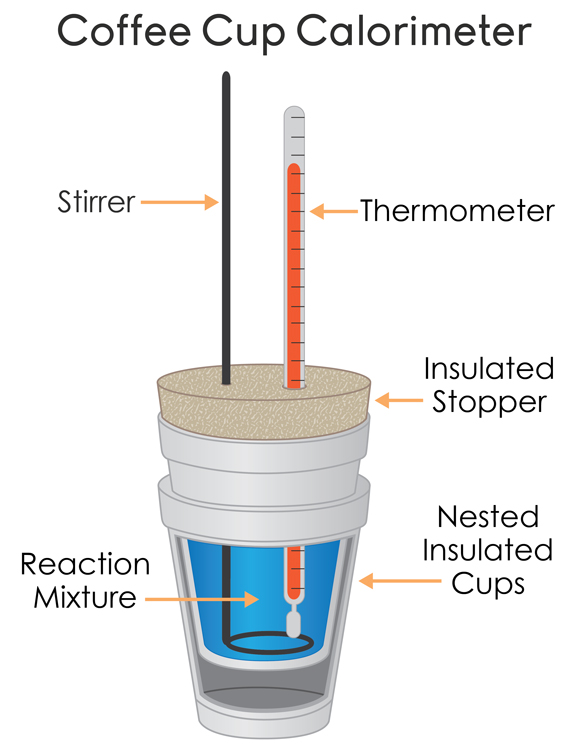
What Is The Importance Of Calorimetry . A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first law of thermodynamics to calorimetry. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. By knowing the change in. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is widely used in chemical reaction and the measuring method of. For example, when an exothermic. Calorimetry is an important method of biological analysis. To do so, the heat is exchanged with a calibrated object. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring.
from socratic.org
By knowing the change in. Calorimetry is widely used in chemical reaction and the measuring method of. Apply the first law of thermodynamics to calorimetry. Calorimetry is an important method of biological analysis. To do so, the heat is exchanged with a calibrated object. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements.
A student conducting a calorimetry investigation determines a negative
What Is The Importance Of Calorimetry Calorimetry is an important method of biological analysis. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements. Apply the first law of thermodynamics to calorimetry. To do so, the heat is exchanged with a calibrated object. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is an important method of biological analysis. For example, when an exothermic. Calorimetry is widely used in chemical reaction and the measuring method of. Calorimetry is used to measure amounts of heat transferred to or from a substance. By knowing the change in.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii What Is The Importance Of Calorimetry In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements. For example, when an exothermic. Compare heat flow from hot to cold objects in an ideal calorimeter. What Is The Importance Of Calorimetry.
From cider.uoregon.edu
Calorimetry Heat Exchange Hot Metal in Cold Water Real and Computer What Is The Importance Of Calorimetry By knowing the change in. Calorimetry is widely used in chemical reaction and the measuring method of. Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry is an important method of biological analysis. A container that prevents heat transfer in or out is called. What Is The Importance Of Calorimetry.
From www.researchgate.net
Basic principle of isothermal titration calorimetry. Schematic What Is The Importance Of Calorimetry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry is widely used in chemical reaction and the measuring method of. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. By knowing the change in. For example, when an exothermic. Calorimetry is used. What Is The Importance Of Calorimetry.
From www.youtube.com
CALORIMETRY EXAMPLE 3 YouTube What Is The Importance Of Calorimetry Calorimetry is an important method of biological analysis. Calorimetry is widely used in chemical reaction and the measuring method of. For example, when an exothermic. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Apply the first law of thermodynamics to calorimetry. Calorimetry is the process of measuring the amount of heat released. What Is The Importance Of Calorimetry.
From engineeringlearn.com
Bomb Calorimeter Definition, Construction, Diagram, Working & Uses What Is The Importance Of Calorimetry Calorimetry is widely used in chemical reaction and the measuring method of. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. For example, when an exothermic. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. To do so, the heat. What Is The Importance Of Calorimetry.
From www.pinterest.com
Calorimetry Lab Report in 2022 Science diagrams, Lab report What Is The Importance Of Calorimetry A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements. Calorimetry is widely used in chemical reaction and the measuring method of. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. To do so, the heat is exchanged with a calibrated. What Is The Importance Of Calorimetry.
From bettyelevisxo.blob.core.windows.net
Fundamentals Of Calorimetry Lab What Is The Importance Of Calorimetry Apply the first law of thermodynamics to calorimetry. Calorimetry is widely used in chemical reaction and the measuring method of. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is used to. What Is The Importance Of Calorimetry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 What Is The Importance Of Calorimetry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Apply the first law of thermodynamics to calorimetry. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is an important method of biological analysis. For example, when an exothermic. By knowing the change in. To. What Is The Importance Of Calorimetry.
From www.blendspace.com
Atomic History Lessons Blendspace What Is The Importance Of Calorimetry In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry is used to measure amounts of heat transferred to or from a substance. A calorimeter is a device used to measure the. What Is The Importance Of Calorimetry.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals What Is The Importance Of Calorimetry Calorimetry is widely used in chemical reaction and the measuring method of. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements. Apply the first law of. What Is The Importance Of Calorimetry.
From socratic.org
A student conducting a calorimetry investigation determines a negative What Is The Importance Of Calorimetry For example, when an exothermic. Apply the first law of thermodynamics to calorimetry. Calorimetry is widely used in chemical reaction and the measuring method of. Calorimetry is used to measure amounts of heat transferred to or from a substance. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. A calorimeter is a device. What Is The Importance Of Calorimetry.
From www.bartleby.com
Answered In the laboratory a "coffee cup"… bartleby What Is The Importance Of Calorimetry Calorimetry is widely used in chemical reaction and the measuring method of. Calorimetry is used to measure amounts of heat transferred to or from a substance. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. What Is The Importance Of Calorimetry.
From schoolbag.info
Figure 7.6. Diagram of a Bomb Calorimeter What Is The Importance Of Calorimetry In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is an important. What Is The Importance Of Calorimetry.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle What Is The Importance Of Calorimetry Apply the first law of thermodynamics to calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is an important method of biological analysis. For example, when an exothermic. In chemistry and thermodynamics, calorimetry (from latin calor. What Is The Importance Of Calorimetry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 What Is The Importance Of Calorimetry For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is an important method of biological analysis. Calorimetry is used to measure amounts of heat transferred to or from a substance. A container that prevents heat transfer in or out is called a calorimeter, and. What Is The Importance Of Calorimetry.
From study.com
Calorimetry Definition, Equation & Types Lesson What Is The Importance Of Calorimetry A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is widely used in chemical reaction and the measuring method of. Calorimetry is used to measure amounts of heat transferred to or from a substance. Apply the first law of thermodynamics to calorimetry. By knowing the change in. For example,. What Is The Importance Of Calorimetry.
From thechemistrynotes.com
Calorimetry Definition, Principle, Types, Application, and Limitations What Is The Importance Of Calorimetry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. To do so, the heat is exchanged with a calibrated object. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is widely used in chemical reaction and the measuring method of. A container. What Is The Importance Of Calorimetry.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts What Is The Importance Of Calorimetry Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is used to measure amounts of heat transferred to or from a substance. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry is widely used in chemical reaction and the measuring method of. By. What Is The Importance Of Calorimetry.